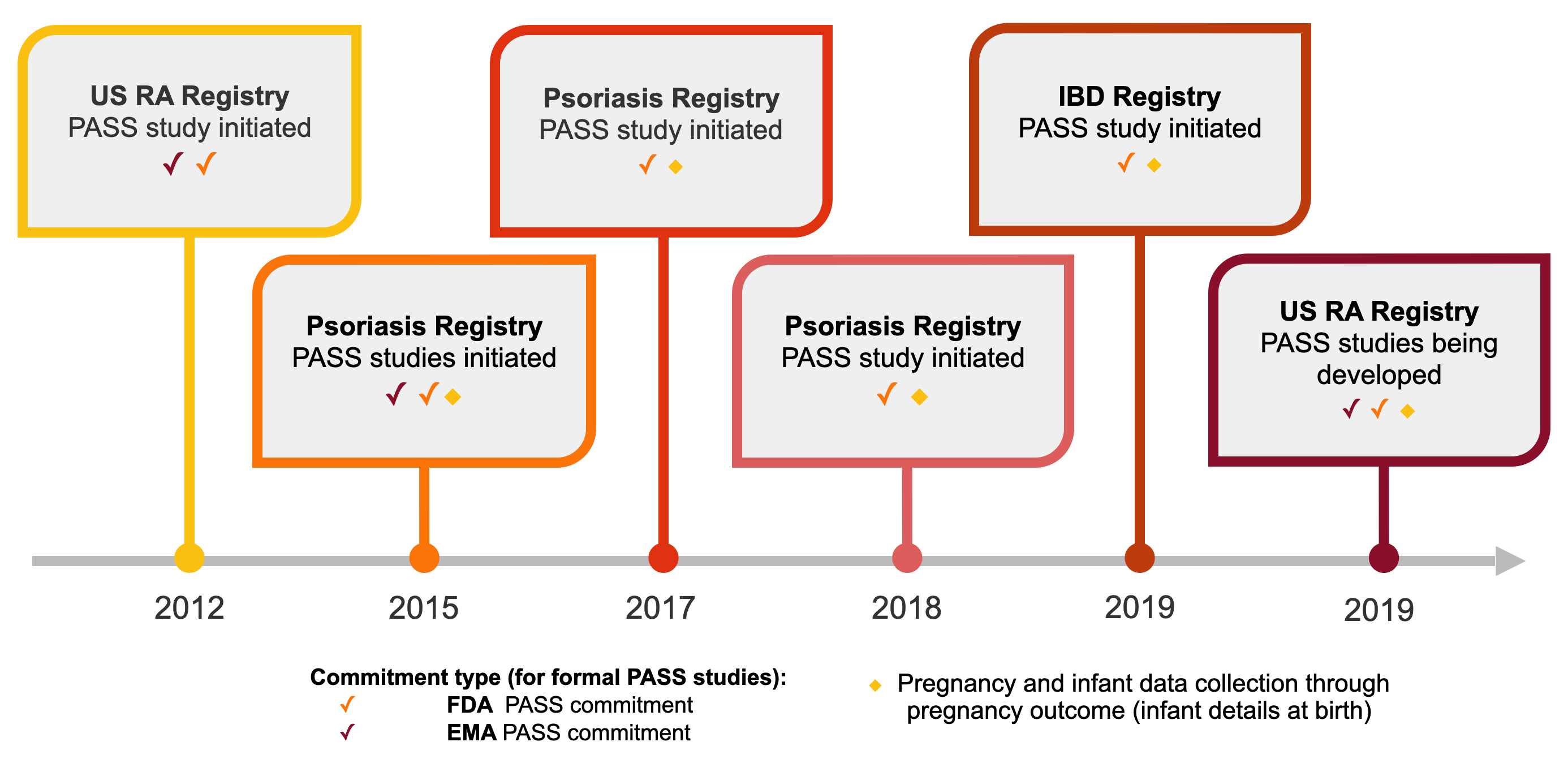
Protocol planning and development support are provided for post-authorization safety studies nested within CorEvitas registries to fulfill U.S. and global commitments and informational needs.
Custom descriptive comparative safety reports are collaboratively developed to address informational needs across stakeholder groups.
Formal analytic comparisons of study cohorts may be completed using a variety of techniques (e.g. propensity score matching or trimming, age and sex standardized rates) to facilitate more formal analytic comparisons.
Robust, established framework for rapid identification and validation of site-reported adverse events can be implemented at defined frequencies per collaboratively developed safety reporting plans.
Experienced safety staff create individual case safety reports regarding serious adverse events or other targeted adverse events based on post-authorization requirements.
Trained specialists and case adjudication committees review and validate physician-reported safety endpoints of special interest.
Fulfill regulatory-mandated pregnancy registries, leveraging robust sample sizes, personalized data collection, access to real-time participants’ data, end-to-end system security protocols, and concierge participant engagement.